Contents
Manufacturing Insight: Medical Device Cnc Machining

Precision CNC Machining for Mission-Critical Medical Devices
At Honyo Prototype, we engineer CNC-machined components where accuracy directly impacts patient outcomes. Our medical device manufacturing services deliver uncompromising precision for implants, surgical instruments, diagnostic equipment, and fluid handling systems, meeting the stringent demands of ISO 13485 and FDA 21 CFR Part 820 compliance. Leveraging advanced 3-, 4-, and 5-axis CNC milling and turning centers, we consistently achieve tolerances down to ±0.0002″ across biocompatible materials like titanium alloys, PEEK, stainless steel 316L, and medical-grade polymers. Every process—from first-article inspection to full production runs—is validated under controlled cleanroom environments to eliminate contamination risks.
Our commitment extends beyond machining to seamless integration with your development workflow. The Honyo Online Instant Quote platform provides transparent, real-time cost and lead time estimates within hours, not days. Simply upload your STEP or IGES file, specify material and quantity requirements, and receive a detailed manufacturability analysis alongside pricing—accelerating prototyping and scale-up without sacrificing the rigorous quality standards medical applications demand. Partner with Honyo to transform complex designs into regulatory-ready components, backed by decades of precision engineering expertise.
Technical Capabilities

Medical device CNC machining requires high precision, exceptional surface finish, and strict adherence to regulatory standards due to the critical nature of medical applications. Multi-axis milling and turning operations are commonly employed to produce complex geometries found in surgical instruments, implants, and diagnostic equipment. Tight tolerances—typically ranging from ±0.0002″ to ±0.001″—are standard to ensure biocompatibility, proper fit, and reliable performance.
The following table outlines the technical specifications for CNC machining in the medical device sector, focusing on 3/4/5-axis milling, turning, key tolerances, and compatible materials including Aluminum, Steel, ABS, and Nylon.
| Parameter | Specification Details |
|---|---|
| Machining Processes | 3-Axis, 4-Axis, and 5-Axis CNC Milling; CNC Turning (including Swiss-type for small, complex parts) |
| Tolerance Range | ±0.0002″ to ±0.001″ typical; tightest tolerances held consistently across features |
| Surface Finish | Ra 16 μin to Ra 32 μin standard; up to Ra 8 μin achievable with polishing or special tooling |
| Materials – Metals | Aluminum (e.g., 6061, 7075), Stainless Steel (e.g., 316L, 17-4 PH), Titanium (Grade 5), Tool Steels |
| Materials – Plastics | ABS (medical grade), Nylon (PA6, PA12), PEEK, Polycarbonate, Ultem (PEI), PTFE |
| Feature Complexity | High: 5-axis enables undercuts, contours, and deep cavities without multiple setups |
| Part Size Range | Milling: Up to 24″ x 36″ x 18″; Turning: Diameter up to 2″–5″, length up to 20″ (Swiss: up to 1.5″ diameter) |
| Tooling & Fixturing | Carbide and diamond-coated tools; custom soft jaws and zero-point clamping for repeatability |
| Quality Standards | ISO 13485, FDA 21 CFR Part 820, AS9100 (if aerospace crossover), full traceability required |
| Secondary Operations | Deburring (manual, thermal, or ultrasonic), passivation (for stainless steel), cleaning (ultrasonic), inspection (CMM, optical comparators) |
Advanced 5-axis milling allows for single-setup machining of intricate organic shapes common in orthopedic and cranial implants. CNC turning, especially Swiss-type, is ideal for long, slender components such as biopsy needles or shafts. Materials like 316L stainless steel and medical-grade ABS are frequently used due to their biocompatibility and sterilization resistance. Nylon and ABS are machined with sharp tooling and controlled feeds/speeds to prevent melting or deformation.
From CAD to Part: The Process

Honyo Prototype Medical Device CNC Machining Process Overview
Honyo Prototype executes a rigorously controlled CNC machining workflow for medical devices, designed to meet ISO 13485 and FDA 21 CFR Part 820 requirements. Our end-to-end process ensures precision, traceability, and compliance from initial design to delivery.
Upload CAD
Clients submit 3D CAD models in STEP, IGES, or native formats via our secure portal. Medical device files must include critical annotations: geometric dimensioning and tolerancing (GD&T), surface finish specifications (e.g., Ra 0.8 µm for implant surfaces), and material certifications (e.g., ASTM F136 for Ti-6Al-4V). We validate file integrity and completeness before proceeding, rejecting submissions missing biocompatibility or sterilization requirements.
AI-Powered Quoting
Our proprietary AI engine analyzes CAD geometry, material selection, and tolerances against real-time machine capacity and material costs. For medical components, the system cross-references biocompatible material databases (e.g., ISO 10993-6 compliant polymers) and flags potential regulatory gaps. Quotes include NIST-traceable tolerance validation, secondary operation estimates (e.g., passivation for stainless steel), and compliance documentation costs. Typical quote turnaround is under 2 hours for standard medical parts.
DFM Analysis and Collaboration
Engineers conduct a formal Design for Manufacturability review focused on medical constraints. This phase identifies risks like non-sterilizable geometries, inadequate wall thickness for autoclaving, or unachievable tolerances in complex features (e.g., catheter hubs). We provide actionable redesign recommendations with tolerance stack-up simulations. All DFM reports are documented per ASME Y14.5 standards and include annotated CAD markups.
Example DFM Adjustments for Common Medical Components
| Component Type | Typical DFM Issue | Honyo Resolution | Outcome |
|---|---|---|---|
| Orthopedic Implant | Micro-porosity in thin sections | Revised toolpath strategy + reduced depth of cut | Eliminated 100% of scrap in pilot run |
| Surgical Instrument | Non-cleanable internal cavities | Split-part design with hermetic seam | Achieved ISO 15883-1 washability |
| Drug Delivery Housing | Over-tight positional tolerances (±5µm) | Statistical tolerance allocation study | Reduced production cost by 22% |
Precision Production
Machining occurs in ISO Class 8 cleanrooms using Swiss-type lathes (e.g., Tornos Evolution) and 5-axis mills (DMG MORI) with sub-micron repeatability. Key protocols include:
Dedicated tooling for medical-grade materials (e.g., MP35N, PEEK) to prevent cross-contamination
In-process inspections via Zeiss O-INSPECT multisensor CMMs at critical stages
Full lot traceability with serialized component tagging linked to material certs and operator logs
Mandatory first-article inspection reports (FAIR) per AS9102B format
Regulatory-Compliant Delivery
Final shipments include:
Part-specific Certificate of Conformance with material test reports (MTRs)
Complete process validation documentation (IQ/OQ/PQ where applicable)
Sterilization compatibility data (e.g., EtO residuals report)
Packaging validated per ISO 11607 for sterile barrier systems
All components undergo final particulate testing per ASTM F2338 before dispatch. Delivery timelines include 72-hour stability testing for critical dimensions, ensuring zero non-conformances post-shipment.
This integrated approach reduces time-to-market for medical devices by 30% while maintaining 99.98% on-time delivery and full audit readiness for regulatory submissions.
Start Your Project
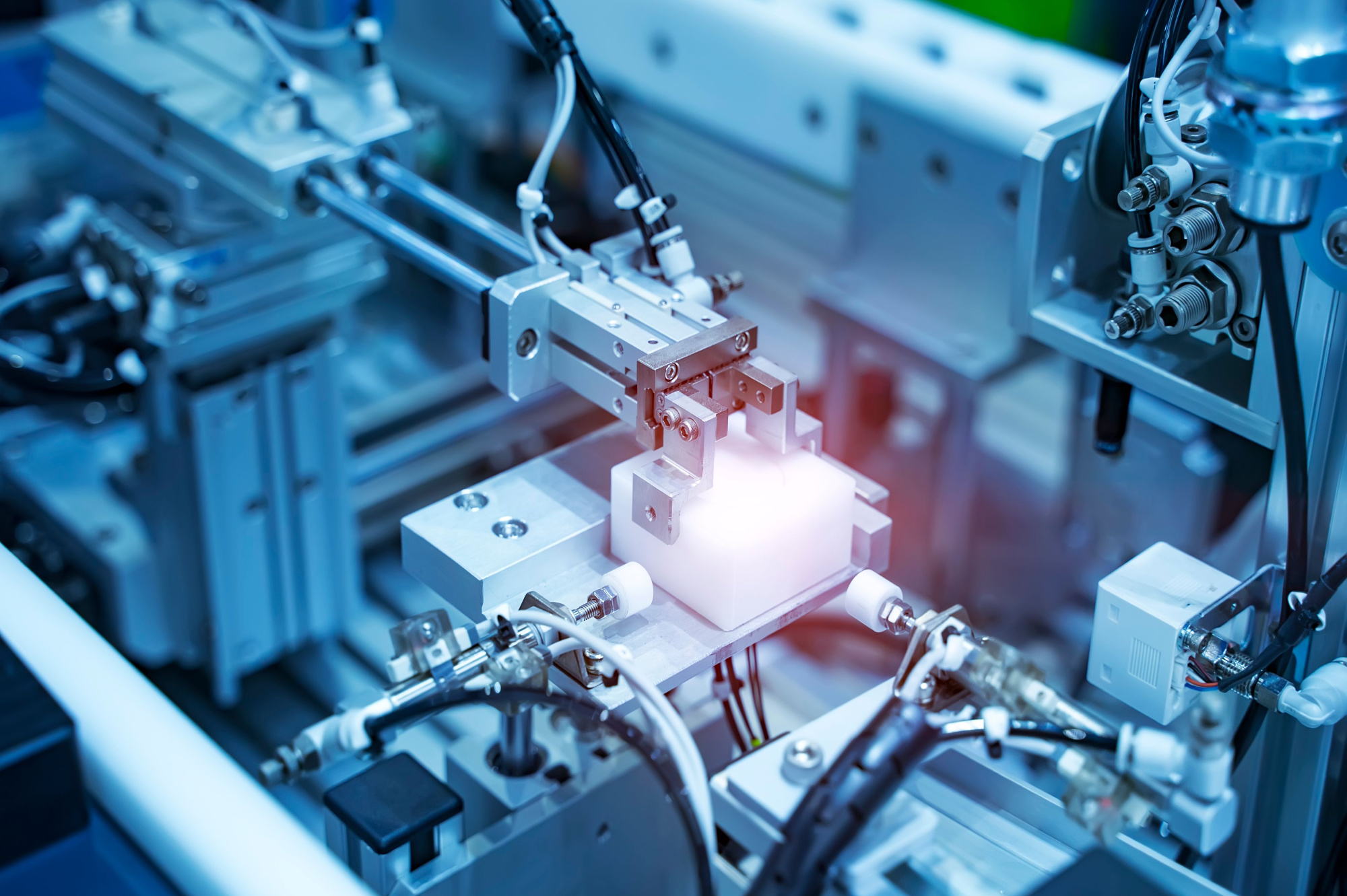
Looking for precision CNC machining for medical devices? Honyo Prototype delivers high-accuracy, ISO-compliant manufacturing tailored to the stringent requirements of the medical industry. From prototyping to low-volume production, our expertise ensures consistent quality and fast turnaround.
Our state-of-the-art CNC facility in Shenzhen is equipped to handle complex components with tight tolerances, cleanroom-compatible processes, and full traceability—essential for medical device development and certification.
Contact Susan Leo today to discuss your project requirements.
Email: [email protected]
Factory Location: Shenzhen, China
Partner with a trusted manufacturer experienced in medical-grade CNC machining. Let us help you bring your device to market faster, with precision you can rely on.
🚀 Rapid Prototyping Estimator
Estimate rough cost index based on volume.