Contents
Manufacturing Insight: Cnc Medical Equipment

Precision CNC Machining for Critical Medical Equipment
Medical device manufacturers face uncompromising demands for dimensional accuracy, material biocompatibility, and regulatory compliance in every component. At Honyo Prototype, we specialize in high-precision CNC machining services engineered explicitly for the medical equipment sector, where tolerances measured in microns directly impact patient safety and device efficacy. Our ISO 13485-certified processes ensure rigorous adherence to quality management standards, from prototyping to low-volume production runs of surgical instruments, implantable components, diagnostic device housings, and fluid handling systems.
We machine complex geometries in advanced materials including medical-grade titanium, PEEK, stainless steel 316L, and engineered polymers, leveraging 5-axis milling and turning capabilities to achieve surface finishes and repeatability essential for FDA and CE-marked devices. Unlike generic machine shops, our engineering team collaborates early in your design phase to optimize manufacturability while mitigating risks associated with sterilization compatibility, fatigue resistance, and cleanroom assembly requirements.
Accelerate your development timeline with Honyo’s Online Instant Quote system. Upload CAD files in STEP, IGES, or native formats to receive a detailed, geometry-aware cost and lead time estimate within hours—not days—enabling faster iteration and procurement decisions without compromising on the traceability or documentation your quality system demands.
| Key Capability | Medical Industry Application |
|---|---|
| ±0.0002″ Tolerance | Implant interfaces, microfluidic channels |
| ISO 13485 Cleanroom Machining | Sterile surgical tool components |
| Exotic Material Expertise | MRI-compatible titanium frames, PEEK spinal cages |
| Full Documentation Traceability | DHR/DHF-compliant production records |
Partner with Honyo Prototype to transform precision-engineered concepts into validated medical equipment components, backed by responsive engineering support and transparent quoting that aligns with your regulatory and operational deadlines.
Technical Capabilities

The following technical specifications outline key performance parameters and capabilities for CNC medical equipment used in the precision machining of components for the medical device industry. Emphasis is placed on 3-axis, 4-axis, and 5-axis milling systems as well as CNC turning centers, with a focus on tight-tolerance production required for surgical instruments, implants, and diagnostic equipment. These machines are commonly used to process materials such as aluminum, stainless steel, ABS, and nylon, which are frequently specified in medical applications due to biocompatibility, strength, and sterilization resistance.
| Parameter | 3-Axis Milling | 4-Axis Milling | 5-Axis Milling | CNC Turning |
|---|---|---|---|---|
| Positioning Accuracy | ±0.005 mm | ±0.005 mm | ±0.003 mm | ±0.005 mm |
| Repeatability | ±0.002 mm | ±0.002 mm | ±0.001 mm | ±0.002 mm |
| Typical Tolerance Range | ±0.010 mm | ±0.008 mm | ±0.005 mm | ±0.010 mm |
| Spindle Speed (max) | 24,000 rpm | 24,000 rpm | 30,000 rpm | 8,000 rpm |
| Spindle Taper | BT30 or HSK-63 | BT30 or HSK-63 | HSK-63 or HSK-40 | Capto C8 or similar |
| Axis Travel (X/Y/Z) | 500 x 400 x 300 mm | 500 x 400 x 300 mm | 400 x 300 x 250 mm | Max. swing 300 mm, 500 mm bed length |
| Rotary Axis (A or B) | N/A | ±360° (Indexing/Continuous) | Dual rotary (A/B or trunnion), ±360° | C-axis (live tooling capable) |
| Control System | Fanuc, Siemens, or Heidenhain | Fanuc, Siemens, or Mitsubishi | Heidenhain or Siemens 840D | Fanuc or Siemens 840D |
| Tool Magazine Capacity | 20–30 tools | 30–40 tools | 40–60 tools | 8–12 live tool positions |
| Coolant System | High-pressure through-spindle | High-pressure through-spindle | High-pressure, mist/air options | Internal/external coolant |
| Materials Processed | Aluminum, Steel, ABS, Nylon | Aluminum, Steel, ABS, Nylon | Aluminum, Titanium, PEEK, Nylon | Stainless Steel, Aluminum, ABS |
| Surface Finish (typical) | Ra 0.8 µm | Ra 0.8 µm | Ra 0.4 µm | Ra 0.8 µm |
| Applications in Medical | Enclosures, jigs, fixtures | Housings, connectors | Implants, surgical tools | Shafts, screws, cannulas |
Notes:
5-axis milling systems provide superior access and accuracy for complex geometries such as cranial implants or joint replacement components. CNC turning with live tooling enables mill-turn operations ideal for small, high-precision parts like bone screws or fluid control valves. Materials such as medical-grade stainless steel (e.g., 316L), aluminum 6061-T6, ABS USP Class VI, and nylon 12 are routinely machined to meet ISO 13485 quality standards. Tight tolerances down to ±5 µm are achievable with thermal compensation, in-process probing, and high-stability machine structures.
From CAD to Part: The Process
Honyo Prototype CNC Medical Equipment Manufacturing Process
Honyo Prototype executes a rigorously controlled workflow for CNC machining medical equipment, ensuring compliance with ISO 13485, FDA 21 CFR Part 820, and biocompatibility standards. Our process integrates automation with engineering oversight to mitigate risks inherent in medical device production. Below is a technical breakdown of each phase.
CAD Upload and Validation
Clients initiate the process by uploading native CAD files (STEP, Parasolid, or native MCAD formats) via our secure customer portal. Our system performs automated validation checks for file integrity, unit consistency, and geometric completeness. Critical medical-specific parameters—such as surface finish callouts per ASTM F86, material traceability requirements, and sterilization compatibility annotations—are flagged for immediate review. Incomplete GD&T or missing biocompatibility specifications (e.g., ISO 10993-1) trigger an automated request for clarification before proceeding.
AI-Powered Quoting with Medical Compliance Screening
Our AI quoting engine, trained on 15,000+ medical device projects, analyzes the validated CAD model to generate a preliminary quote within 2 hours. The AI cross-references material databases for USP Class VI or ISO 10993-certified polymers (e.g., PEEK, Ultem) and implant-grade metals (Ti-6Al-4V ELI, 316LVM stainless steel). Simultaneously, it scans for design elements requiring special validation:
Tight tolerances (< ±0.005mm) affecting functional performance
Undercuts complicating sterilization validation
Surface roughness parameters impacting bioburden retention
The output includes cost drivers, lead time estimates, and a preliminary risk assessment report. A Senior Manufacturing Engineer reviews all AI outputs to confirm regulatory alignment before client submission.
Medical-Grade DFM Analysis
Design for Manufacturability (DFM) review is conducted by ASQ-certified engineers specializing in medical devices. This phase focuses on eliminating production risks that could compromise device safety or efficacy. Key evaluation criteria include:
| DFM Parameter | Medical-Specific Requirement | Honyo Mitigation Action |
|---|---|---|
| Wall Thickness | Minimum 0.5mm for autoclave sterilization integrity | Recommend ribbing or coring to prevent warpage |
| Edge Break | 0.127mm max radius per FDA guidance for implant interfaces | Specify controlled break via CNC chamfer routine |
| Material Removal Rate | Avoid thermal degradation of polymers during machining | Optimize toolpaths for 40% lower RPM than commercial parts |
| Parting Lines | Eliminate seams in fluid-contact zones (e.g., IV connectors) | Redesign for single-setup machining |
The DFM report includes annotated CAD markups, validation test recommendations (e.g., ISO 11607 seal strength testing), and formal documentation for DHF traceability.
Precision Production in Controlled Environments
Production occurs in ISO Class 7 and 8 cleanrooms with environmental monitoring (particulate counts, temperature/humidity logs). All CNC equipment undergoes daily calibration per NIST standards, with tool wear compensated via in-process probing. Critical controls include:
Dedicated tooling for medical-grade materials to prevent cross-contamination
100% in-process inspection of critical features using Zeiss CMMs with SPC tracking
Real-time machining data logging for full lot traceability (material certs, operator IDs, machine parameters)
Post-processing per ASTM F86 for passivation (stainless steel) or electropolishing (implants)
Regulatory-Compliant Delivery
Final delivery includes the machined components with:
Complete Device History Record (DHR) package: material traceability, inspection reports, process validation summaries
Certificate of Conformance referencing ISO 13485:2016 and client-specific specifications
Biocompatibility documentation (if applicable) with lot-specific test results
Sterilization compatibility statement per AAMI ST79
All shipments use validated packaging per ISO 11607, with environmental data loggers for temperature-sensitive devices. Honyo provides electronic DMR access via our QMS portal for seamless integration into client regulatory submissions.
This end-to-end process ensures medical equipment meets stringent safety requirements while reducing time-to-market by 30% compared to traditional prototyping vendors through embedded compliance controls.
Start Your Project
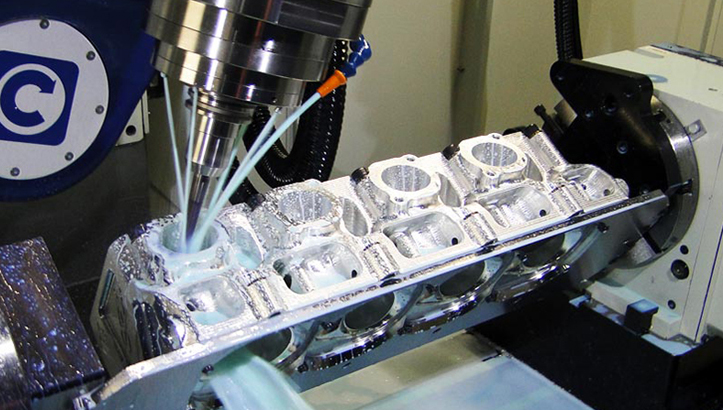
Looking for precision CNC medical equipment manufactured to the highest industry standards? Honyo Prototype specializes in high-accuracy CNC machining for medical devices and equipment, ensuring reliability, biocompatibility, and strict adherence to regulatory requirements.
Our state-of-the-art facility in Shenzhen, China, supports rapid prototyping and scalable production runs with full traceability and ISO-compliant processes.
Contact Susan Leo today to discuss your medical device manufacturing needs.
Email: [email protected]
Trust Honyo Prototype for precision, quality, and on-time delivery in medical equipment manufacturing.
🚀 Rapid Prototyping Estimator
Estimate rough cost index based on volume.